Research Description
The Hoover Lab aims to elucidate the mechanisms of transition metal catalyzed organic transformations in order to understand and improve upon reactions relevant to current societal challenges, in particular those related to health, energy, and the environment.
Catalytic methods have widespread applications ranging from the large scale synthesis of commodity chemicals to the fine chemical and pharmaceutical industries. Many of today's societal challenges, including those related to health, energy, and the environment, will be solved with with catalytic strategies. Solutions to these challenges require a fundamental understanding of the catalysts and their mechanisms. In the Hoover Group, we map the mechanisms of organic transformations catalyzed by transition-metal complexes. We are particularly interested in catalytic redox reactions in which bond-breaking and -forming steps occur along with oxidation or reduction steps. We develop new organic reactions, synthesize and characterize organometallic intermediates, and measure reaction kinetics. Researchers in our group are trained in synthetic organic techniques, organometallic synthesis, homogeneous catalysis, spectroscopy, and kinetics.
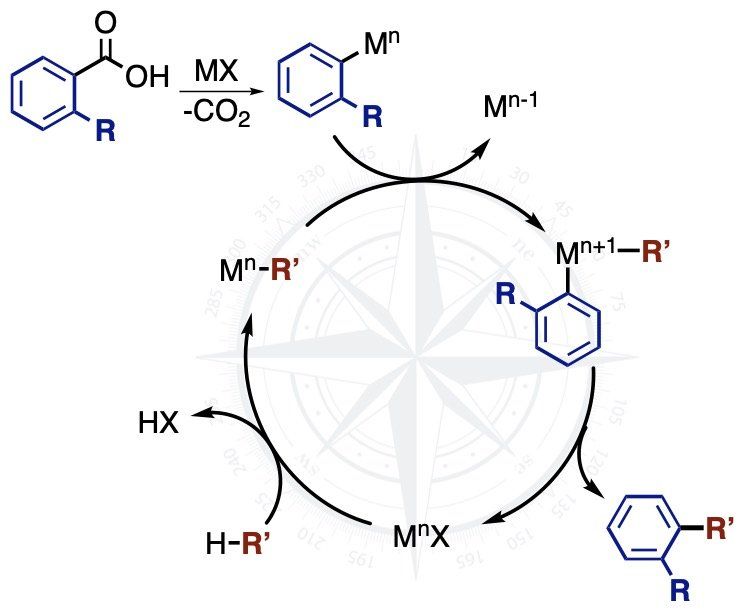
Understanding Metal-Mediated Decarboxylation Reactions
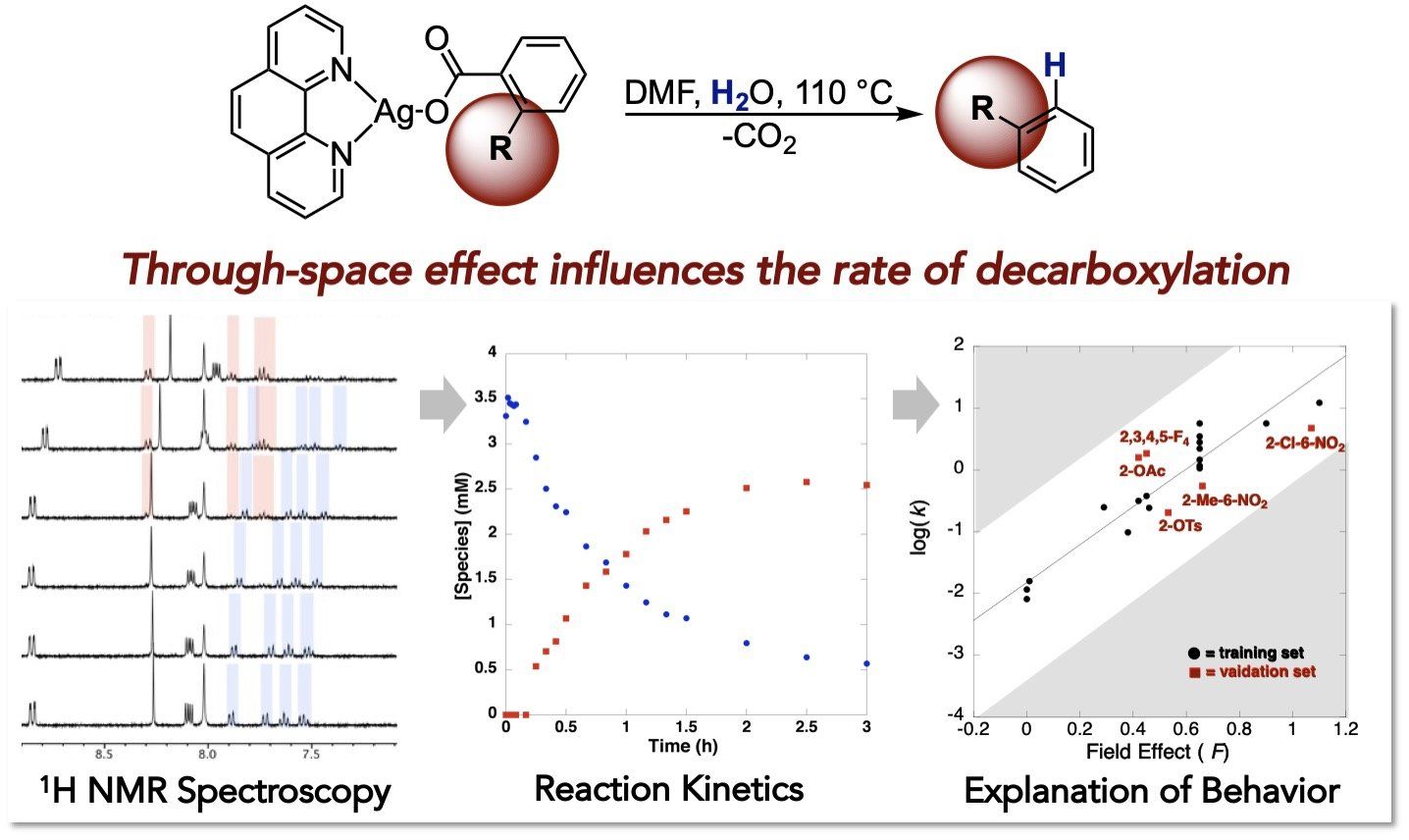
A decarboxylation reaction of carboxylic acids allows us to use benzoic acids a coupling partners to generate more complex products through coupling reactions. This strategy has many advantages since benzoic acids are cheaper and more stable than typical organometallic coupling partners. There are some challenges, however, and decarboxylation reactions are often limited to specific classes of benzoic acids, such as ortho-nitrobenzoic acids or perflurobenzoic acids. Our most recent work in this area has uncovered the origin of this ‘ortho-nitro effect’ and shown that the rates of decarboxylation correlate with the field effect (F), a through-space influence, of the benzoate substituents.
Oxidative Decarboxylative Arylation Reactions
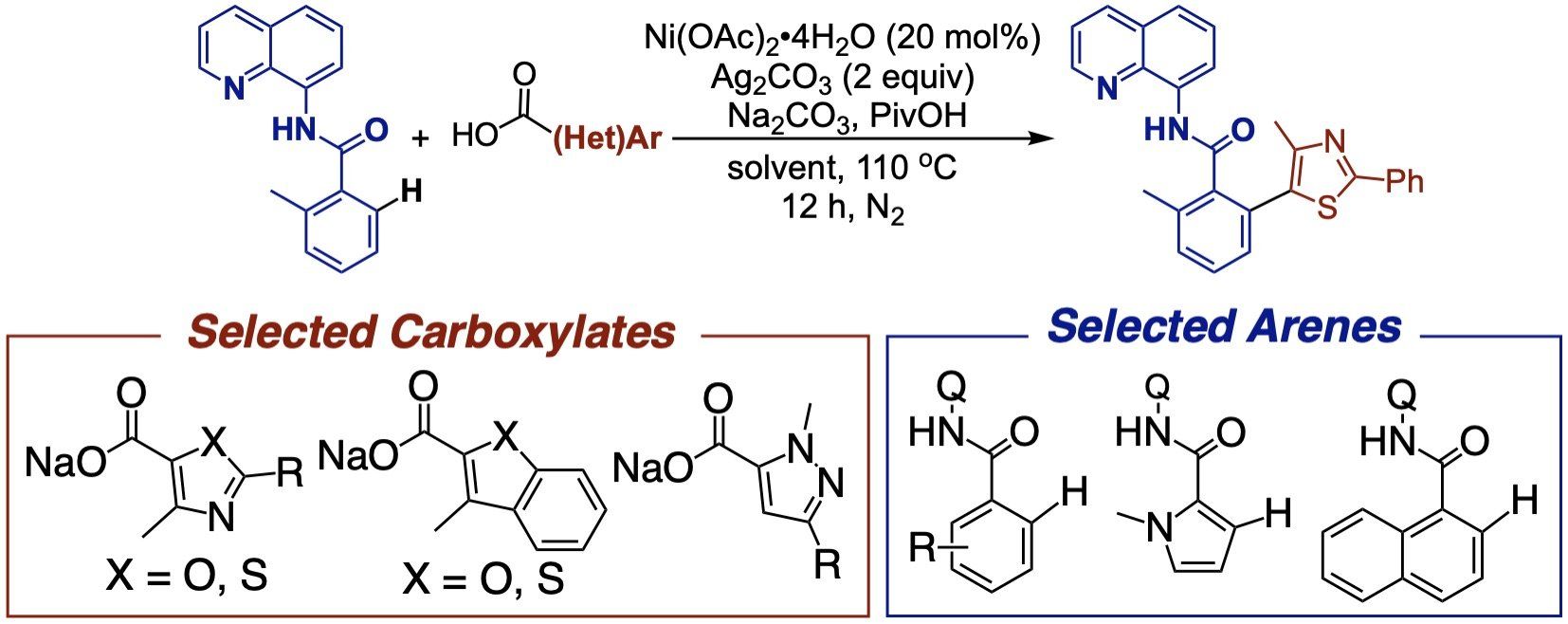
The decarboxylative coupling of benzoic acids with arene C-H bonds would provide an efficient route to biaryl and heterobiaryl products. We have developed several oxidative decarboxylative arylation reactions using either copper or nickel catalysts. Most recently, we have developed a new nickel-catalyzed decarboxylative arylation reaction that is capable of overcoming the traditional ‘ortho-nitro’ limitations and now enables the efficient coupling of a large scope of heteroaromatic carboxylates.
Oxidative Decarboxylative Thiolation Reactions
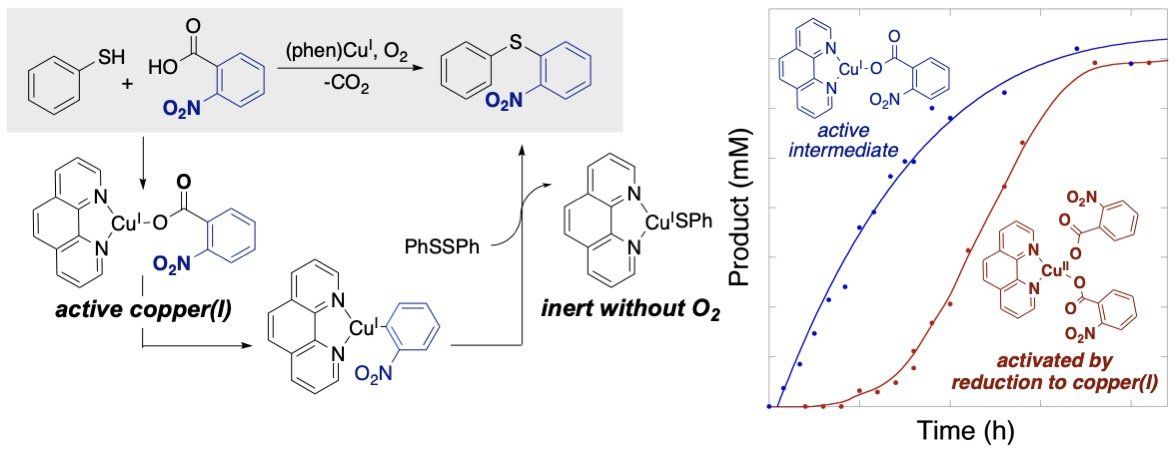
We are also interested in forming new carbon-heteroatom bonds with our oxidative decarboxylative coupling (ODC) reactions and have developed a new aerobic copper-catalyzed reaction of this type to couple thiophenols with benzoic acid. This reaction is unique in it’s ability to use air (oxygen) as the oxidant, while most ODC reactions rely on silver salt oxidants. Our recent work in this area has explored the mechanism of this reaction and established a surprising role for copper(I) intermediates despite the oxidizing conditions that might be expected to readily form copper(II) species.
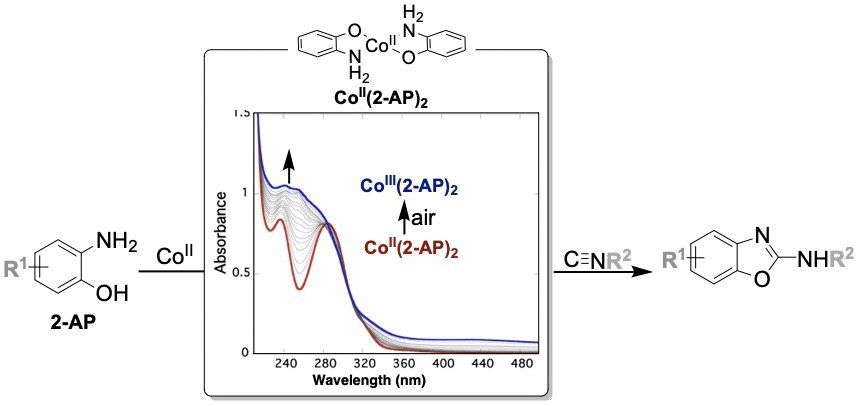
Cobalt Catalyzed Aerobic Oxidation Reactions
Cobalt Catalyzed Aerobic Oxidation Reactions
We are also developing new cobalt-catalyzed reactions that utilize air (oxygen) as the terminal oxidant. In many cases, simple cobalt salts alone aren’t capable of efficiently using oxygen for the selective oxidation of organic molecules. Instead, redox mediators are often needed. We have found that aminophenols and related compounds are able to act as both redox mediator and coupling partner to enable new Co-catalyzed aerobic transformations. Most recently, we have reported the oxidative cyclization of aminophenols with isontriles to generate substituted benzoxazole products.
Hear About Some of Our Research
2018 CCHF Virtual Symposium
2023 WVU Benedum Schowcase
Video Tour of WVU Labs
All Rights Reserved | The Hoover Lab